Symbol Of An Alpha Particle
seoindie
Sep 10, 2025 · 7 min read
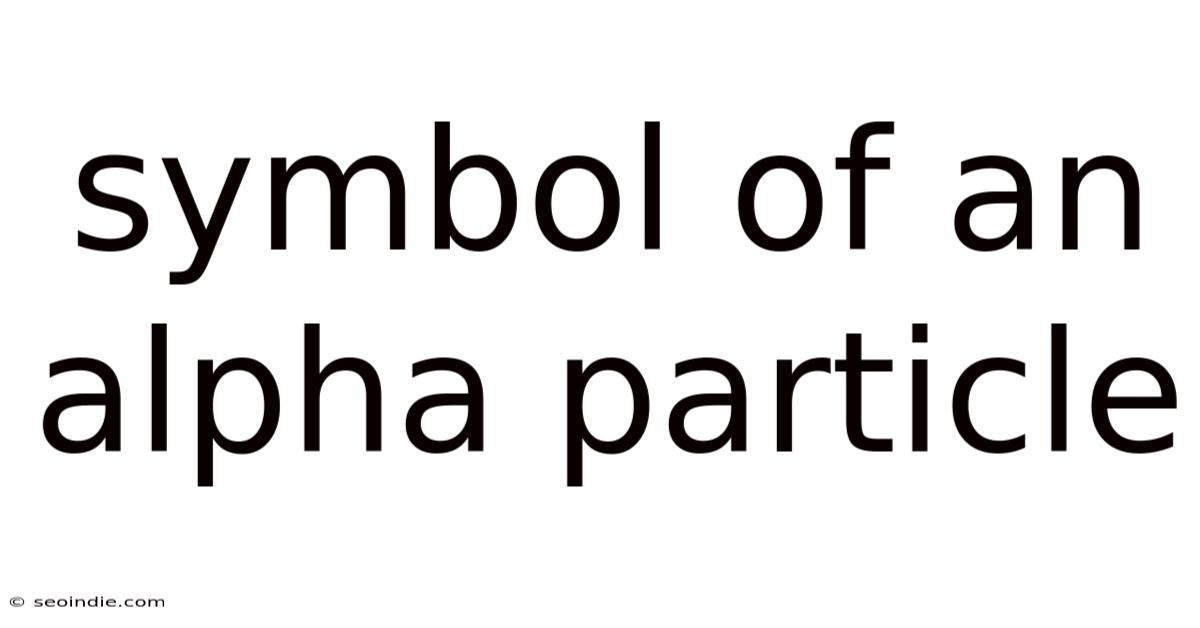
Table of Contents
Unveiling the Symbol of an Alpha Particle: A Deep Dive into Radioactive Decay
The alpha particle, a fundamental component in the realm of nuclear physics and radioactive decay, holds a significant place in our understanding of atomic structure and nuclear processes. Understanding its symbol is crucial for interpreting nuclear reactions and comprehending the nature of radioactivity itself. This article delves deep into the symbol of an alpha particle, exploring its representation, its significance in nuclear equations, and its connection to the particle's properties and behavior. We will also explore its historical context and its importance in various scientific applications.
Introduction: Deciphering the Alpha Particle's Identity
The alpha particle, symbolized as α, is a highly energetic particle emitted during alpha decay, a type of radioactive decay. It's essentially a helium nucleus, consisting of two protons and two neutrons tightly bound together. This unique composition gives it distinct properties, which are reflected in its symbol and its behavior during nuclear reactions. Understanding this symbol and what it represents is foundational to grasping the concepts of radioactivity and nuclear physics. We will unpack the meaning behind the symbol and explore how it helps us understand the nature of alpha particles and their role in radioactive decay processes.
The Symbol α: A Simple Yet Powerful Representation
The simple Greek letter alpha (α) represents a complex entity. While it might seem unassuming, this symbol efficiently encapsulates the key characteristics of the alpha particle: its composition and its charge. The symbol itself doesn’t explicitly state the number of protons and neutrons, but its use within the context of nuclear equations implicitly conveys this information. This concise representation is crucial for simplifying complex nuclear reactions and making them readily understandable.
Understanding the Context: Alpha Decay and Nuclear Equations
Alpha decay occurs when an unstable atomic nucleus spontaneously ejects an alpha particle. This process reduces the atomic number (number of protons) of the parent nucleus by two and the mass number (total number of protons and neutrons) by four. The symbol α is crucial in depicting this transformation within nuclear equations.
Let's consider a typical alpha decay equation:
²³⁸U₉₂ → ²³⁴Th₉₀ + ⁴He₂
In this equation:
- ²³⁸U₉₂ represents the parent nucleus, uranium-238, with a mass number of 238 and an atomic number of 92.
- ²³⁴Th₉₀ represents the daughter nucleus, thorium-234, with a mass number of 234 and an atomic number of 90.
- ⁴He₂ represents the alpha particle, with a mass number of 4 and an atomic number of 2. This is often simplified to just α in many equations.
The equation demonstrates the conservation of mass number and atomic number during the decay process. The mass numbers on both sides (238 = 234 + 4) and the atomic numbers (92 = 90 + 2) are balanced, illustrating a fundamental principle of nuclear reactions. The alpha particle's symbol, α, plays a vital role in ensuring this balance is clearly represented.
Alpha Particle Properties and Their Reflection in the Symbol
While the symbol α doesn't explicitly list all the particle's properties, it implicitly points towards several key characteristics:
-
Charge: The alpha particle carries a +2 charge, stemming from its two protons. This positive charge is implied by its place in nuclear equations and its interaction with electric and magnetic fields. Its positive charge is crucial for understanding its interactions with matter and its ability to ionize atoms.
-
Mass: The alpha particle has a mass approximately four times that of a proton or neutron. This relatively large mass contributes to its lower penetration power compared to beta or gamma radiation. While the symbol doesn't show the mass explicitly, the mass number (4) in the complete representation ⁴He₂ clearly indicates the mass.
-
Composition: The alpha particle's composition of two protons and two neutrons is understood implicitly through its symbol's use in nuclear equations and its association with a helium nucleus. This composition is crucial for understanding its stability and interaction with other particles.
-
Penetration Power: The alpha particle's relatively large mass and charge mean it has a relatively low penetrating power. It can be stopped by a sheet of paper or even a few centimeters of air. This property is not directly conveyed by the symbol itself, but it's directly linked to the particle’s composition and charge.
Historical Context: The Discovery and Symbolism
The discovery of alpha particles was a significant milestone in the history of nuclear physics. Ernest Rutherford, through his gold foil experiment, identified alpha particles as a distinct form of radiation emanating from radioactive substances. The adoption of the Greek letter alpha to symbolize this particle reflects the historical context of its discovery and its role in advancing our understanding of the atom. The use of Greek letters to denote different types of radiation (alpha, beta, gamma) became a standard convention in physics, signifying a historical development and consistency in nomenclature.
Beyond the Symbol: Applications of Alpha Particles
The understanding of alpha particles, symbolized by α, has numerous applications across various scientific fields:
-
Radioactive Dating: The predictable decay rate of certain alpha-emitting isotopes is used in radiometric dating, providing insights into the age of geological formations and artifacts. Understanding the alpha decay process and its representation are crucial to accurate dating.
-
Smoke Detectors: Many smoke detectors utilize americium-241, an alpha emitter, to detect smoke particles. The alpha particles ionize the air, and changes in ionization current due to smoke trigger the alarm. The symbol α is not explicitly present in the design of the detector, but the understanding of its radioactive properties and decay is essential for its function.
-
Radiation Therapy: While highly damaging to living tissue at close range, focused beams of alpha particles are explored in targeted radiation therapy for cancer treatment. The understanding of their properties, and the ability to precisely represent their interaction within equations, are key for developing effective treatments.
-
Nuclear Research: Alpha particles are used in experiments to probe the structure of atomic nuclei and test theoretical models of nuclear interactions. The consistent use of the symbol, particularly in equations, facilitates communication and analysis within the field.
Frequently Asked Questions (FAQ)
-
Q: What is the difference between α and ⁴He₂?
-
A: Both represent an alpha particle, but ⁴He₂ provides more detailed information: a mass number of 4 and an atomic number of 2. α is a shorthand notation, suitable for most equations where the detailed composition is already implied by the context.
-
Q: Can an alpha particle exist independently outside of a nucleus?
-
A: Yes, an alpha particle is emitted from the nucleus during alpha decay and can exist independently for a period of time. However, it is highly reactive and quickly seeks to become part of a stable atom, often combining with electrons to form a helium atom.
-
Q: Is the alpha symbol always written as α?
-
A: While α is the most common representation, you might occasionally see it written as ⁴He²⁺ to emphasize the charge. However, α remains the standard and most commonly used symbol.
-
Q: Why are Greek letters used to represent radiation types?
-
A: The use of Greek letters (alpha, beta, gamma) for different types of radiation is a historical convention that has become standardized within the field of physics.
Conclusion: The Significance of a Simple Symbol
The seemingly simple symbol α, representing the alpha particle, packs a wealth of information about this crucial component of nuclear physics. Its use in nuclear equations ensures balance and clarity, highlighting the fundamental principles of conservation of mass and charge during radioactive decay. Understanding the symbol and its connection to the alpha particle's properties is essential for grasping concepts related to radioactivity, nuclear reactions, and a variety of scientific applications. From radioactive dating to cancer therapy, the alpha particle's significance is undeniable, and its symbolic representation plays a crucial role in our understanding and application of this fundamental particle. The symbol’s simplicity belies the depth of knowledge it encapsulates and represents a cornerstone of our understanding of the nuclear world.
Latest Posts
Latest Posts
-
Is Miles Longer Than Kilometers
Sep 10, 2025
-
What Percent Is 2 6
Sep 10, 2025
-
Body Tube On A Microscope
Sep 10, 2025
-
Convert 11 Cm To Inches
Sep 10, 2025
-
Proof 2nd Equation Of Motion
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about Symbol Of An Alpha Particle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.