Molar Mass Ba Oh 2
seoindie
Sep 13, 2025 · 6 min read
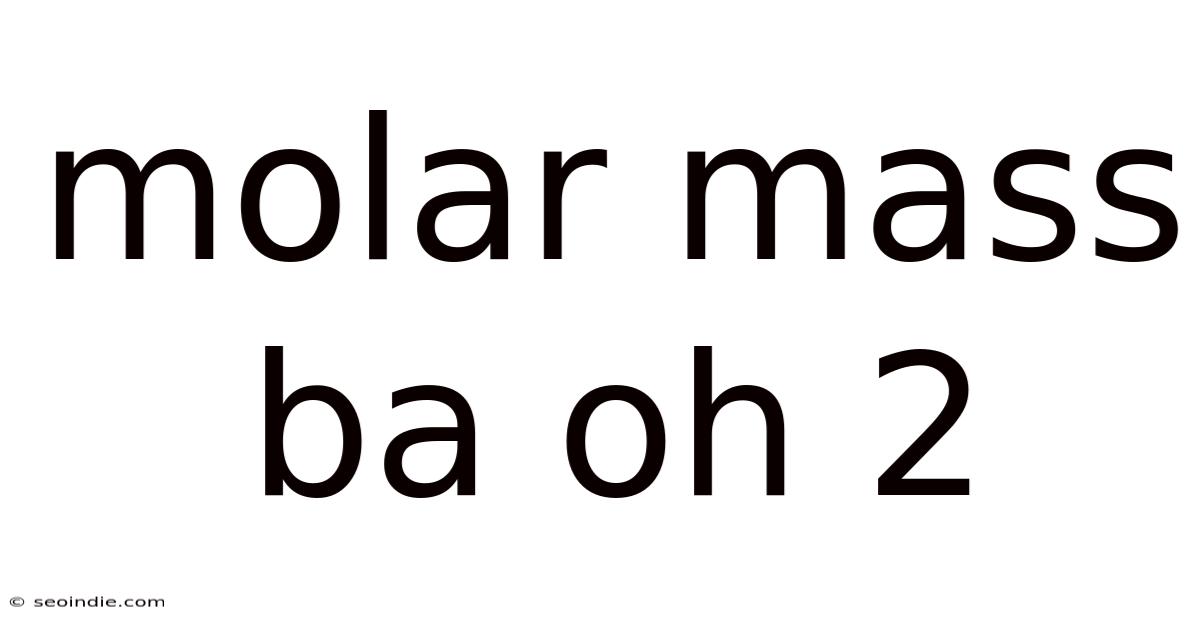
Table of Contents
Understanding Molar Mass: A Deep Dive into Ba(OH)₂
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and applications. This article will delve into the detailed calculation and understanding of the molar mass of barium hydroxide, Ba(OH)₂, exploring the underlying principles and practical implications. We'll unravel the process step-by-step, making it accessible for both beginners and those seeking a refresher on this important chemical concept. By the end, you will have a comprehensive grasp of molar mass calculations and its relevance to stoichiometry and other chemical analyses.
Introduction: What is Molar Mass?
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). Essentially, the molar mass tells us how many grams are in one mole of a particular substance. It's expressed in grams per mole (g/mol). Knowing the molar mass allows us to convert between the mass of a substance and the number of moles, a critical step in many chemical calculations. This is particularly important when dealing with chemical reactions, where the relative amounts of reactants and products are crucial.
For simple elements, the molar mass is simply the atomic mass (found on the periodic table) expressed in grams per mole. For compounds, like Ba(OH)₂, the molar mass is the sum of the molar masses of all the atoms in the chemical formula.
Calculating the Molar Mass of Ba(OH)₂
Barium hydroxide, Ba(OH)₂, is an ionic compound composed of barium (Ba), oxygen (O), and hydrogen (H) atoms. To calculate its molar mass, we need the atomic masses of each element from the periodic table:
- Barium (Ba): Approximately 137.33 g/mol
- Oxygen (O): Approximately 16.00 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
Now, let's break down the calculation:
-
Barium (Ba): There is one barium atom in the formula Ba(OH)₂, so its contribution to the molar mass is 137.33 g/mol.
-
Oxygen (O): There are two oxygen atoms in the formula Ba(OH)₂. Therefore, the contribution from oxygen is 2 * 16.00 g/mol = 32.00 g/mol.
-
Hydrogen (H): There are two hydrogen atoms in the formula Ba(OH)₂. The contribution from hydrogen is 2 * 1.01 g/mol = 2.02 g/mol.
-
Total Molar Mass: To find the total molar mass of Ba(OH)₂, we sum the contributions from each element: 137.33 g/mol + 32.00 g/mol + 2.02 g/mol = 171.35 g/mol
Therefore, the molar mass of Ba(OH)₂ is approximately 171.35 g/mol. This means that one mole of barium hydroxide weighs approximately 171.35 grams.
Significance of Molar Mass in Stoichiometric Calculations
The molar mass of Ba(OH)₂ is crucial for various stoichiometric calculations. Stoichiometry is the branch of chemistry dealing with the quantitative relationships between reactants and products in chemical reactions. Let's look at a few examples:
-
Converting Grams to Moles: If you have a certain mass of Ba(OH)₂, you can use its molar mass to determine the number of moles present. For example, if you have 342.7 grams of Ba(OH)₂, the number of moles would be: (342.7 g) / (171.35 g/mol) = 2 moles.
-
Converting Moles to Grams: Conversely, if you know the number of moles of Ba(OH)₂, you can calculate its mass. If you have 0.5 moles of Ba(OH)₂, its mass would be: (0.5 mol) * (171.35 g/mol) = 85.68 grams.
-
Limiting Reactant Determination: In chemical reactions involving Ba(OH)₂, knowing its molar mass is vital for determining the limiting reactant – the reactant that is completely consumed first and limits the amount of product formed. This calculation often involves converting the mass of each reactant to moles using their respective molar masses.
-
Yield Calculations: Molar mass is also essential for calculating the theoretical yield and percent yield of a reaction. The theoretical yield is the maximum amount of product that can be formed based on stoichiometry, while the percent yield compares the actual yield to the theoretical yield.
Beyond the Basics: Isotopes and Atomic Mass
The atomic masses used in our calculation are average atomic masses, which take into account the natural abundance of different isotopes of each element. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This means they have the same atomic number but different mass numbers. The average atomic mass is a weighted average of the masses of all isotopes, considering their relative abundance in nature.
The periodic table lists these average atomic masses, which are sufficient for most general chemistry calculations. However, for highly precise work, the specific isotopic composition of the sample must be considered.
Practical Applications of Ba(OH)₂ and its Molar Mass
Barium hydroxide, a strong base, finds applications in various fields:
-
Industrial Chemistry: It's used in the sugar industry for the purification of beet sugar, and in the chemical industry for various syntheses and as a reagent in titrations.
-
Analytical Chemistry: Its strong basicity makes it useful in acid-base titrations to determine the concentration of acidic solutions. The precise molar mass is critical for accurate calculations in these titrations.
-
Environmental Remediation: It has been investigated for use in removing pollutants from water and soil. Understanding its molar mass is crucial for determining the necessary amount for effective remediation.
-
Other Applications: It also finds niche applications in the production of other barium compounds and as a component in some specialized lubricants.
Frequently Asked Questions (FAQ)
Q: Can the molar mass of Ba(OH)₂ vary?
A: The molar mass calculated using the average atomic masses from the periodic table is an approximation. Slight variations can occur due to the isotopic composition of the sample, but these differences are usually negligible for most purposes.
Q: Why is it important to use the correct number of significant figures?
A: Using the correct number of significant figures ensures accuracy and reflects the precision of the measurements and calculations. Rounding off prematurely can introduce errors, especially in more complex calculations.
Q: How do I determine the empirical formula if I only know the molar mass and the percentage composition of elements?
A: The percentage composition of each element can be used to determine the empirical formula (the simplest whole-number ratio of atoms in a compound). Once you have the empirical formula, and the molar mass, you can determine the molecular formula (the actual number of atoms of each element in a molecule).
Q: What other compounds require similar molar mass calculations?
A: Many compounds require similar molar mass calculations, including other ionic compounds like sodium chloride (NaCl), potassium hydroxide (KOH), and calcium carbonate (CaCO₃), as well as molecular compounds like water (H₂O), carbon dioxide (CO₂), and glucose (C₆H₁₂O₆).
Conclusion: Mastering Molar Mass Calculations
Understanding and calculating the molar mass of compounds like Ba(OH)₂ is a fundamental skill for any chemist. It underpins various chemical calculations, including stoichiometry, titration, and yield determination. This comprehensive guide has provided a step-by-step approach to calculating the molar mass, emphasized its importance in various chemical contexts, and explored some practical applications of barium hydroxide. By mastering this concept, you will significantly enhance your understanding of chemical quantities and their interactions. Remember to always refer to an updated periodic table for the most accurate atomic masses. The ability to perform these calculations accurately is crucial for success in chemistry and related fields.
Latest Posts
Latest Posts
-
Pic Of A Acute Angle
Sep 13, 2025
-
Large Intestine Of A Frog
Sep 13, 2025
-
Motivational Words Starting With E
Sep 13, 2025
-
What Are Negative Rational Numbers
Sep 13, 2025
-
Factors Of 78 In Pairs
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Ba Oh 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.