G Ml To Kg L
seoindie
Sep 22, 2025 · 6 min read
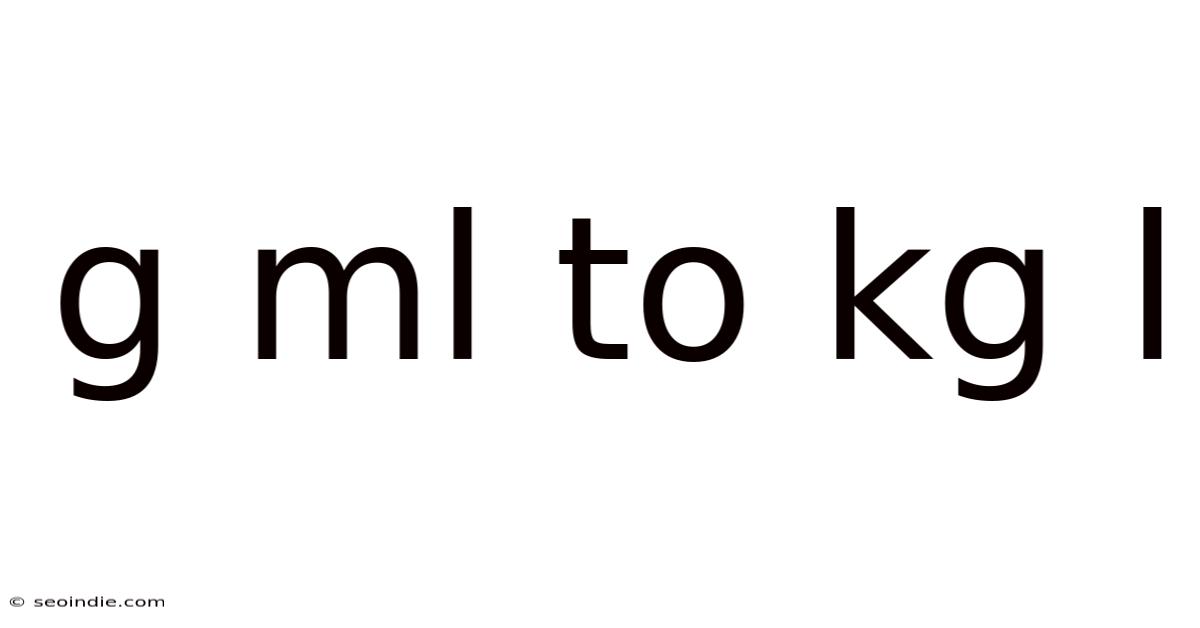
Table of Contents
Understanding the Conversion: Grams, Milliliters, Kilograms, and Liters
Converting between units of mass (grams and kilograms) and units of volume (milliliters and liters) requires a crucial understanding of density. This article will thoroughly explore the relationship between grams (g), milliliters (mL), kilograms (kg), and liters (L), explaining the conversion process and highlighting common pitfalls. Understanding these conversions is essential in various fields, from cooking and baking to chemistry and engineering. We'll delve into the scientific principles, provide step-by-step instructions, and address frequently asked questions to ensure a comprehensive understanding.
Introduction: The Importance of Density
The key to understanding the conversion between grams/kilograms and milliliters/liters lies in the concept of density. Density is defined as the mass per unit volume of a substance. It's typically expressed in grams per milliliter (g/mL) or kilograms per liter (kg/L). The density of a substance is a physical property that is constant under specific conditions (temperature and pressure). Water, for example, has a density of approximately 1 g/mL at 4°C. This means that 1 mL of water has a mass of approximately 1 gram.
However, it's crucial to remember that this 1:1 ratio only applies to water and a few other substances at specific conditions. The density of most substances is different, meaning that 1 mL of a substance will not necessarily weigh 1 gram. To convert between mass and volume, you must know the density of the substance in question.
Step-by-Step Conversion: From Grams to Milliliters and Vice Versa
Let's break down the conversion process. We will use the formula that links mass, volume, and density:
Density = Mass / Volume
We can rearrange this formula to solve for mass or volume:
- Mass = Density x Volume
- Volume = Mass / Density
Example 1: Converting Grams to Milliliters
Let's say we have 50 grams of ethanol, and we want to know its volume in milliliters. The density of ethanol is approximately 0.789 g/mL. Using the formula:
Volume = Mass / Density = 50 g / 0.789 g/mL ≈ 63.37 mL
Therefore, 50 grams of ethanol occupy approximately 63.37 milliliters.
Example 2: Converting Milliliters to Grams
Suppose we have 100 mL of olive oil, and its density is approximately 0.92 g/mL. To find the mass in grams:
Mass = Density x Volume = 0.92 g/mL x 100 mL = 92 g
Therefore, 100 mL of olive oil has a mass of 92 grams.
Step-by-Step Conversion: From Kilograms to Liters and Vice Versa
The conversion between kilograms and liters follows the same principle, but we need to be mindful of the unit conversions. Remember that 1 kilogram (kg) = 1000 grams (g) and 1 liter (L) = 1000 milliliters (mL).
Example 3: Converting Kilograms to Liters
Let's convert 2 kilograms of mercury into liters. The density of mercury is approximately 13.6 g/mL. First, convert kilograms to grams:
2 kg x 1000 g/kg = 2000 g
Now, we use the formula:
Volume (mL) = Mass (g) / Density (g/mL) = 2000 g / 13.6 g/mL ≈ 147.06 mL
Finally, convert milliliters to liters:
147.06 mL / 1000 mL/L ≈ 0.147 L
Therefore, 2 kilograms of mercury occupy approximately 0.147 liters.
Example 4: Converting Liters to Kilograms
We have 0.5 liters of gasoline, and its density is approximately 0.74 kg/L. To find the mass in kilograms:
Mass (kg) = Density (kg/L) x Volume (L) = 0.74 kg/L x 0.5 L = 0.37 kg
Therefore, 0.5 liters of gasoline has a mass of 0.37 kilograms.
Scientific Explanation: Density and its Variations
Density is a fundamental property of matter and is affected by several factors, primarily temperature and pressure. As temperature increases, the volume of a substance usually increases (except for water near its freezing point), leading to a decrease in density. Similarly, changes in pressure can also affect density. For most liquids and solids, the effect of pressure on density is relatively small, but it can be significant for gases.
The density of a mixture or solution depends on the densities and proportions of its components. For example, the density of saltwater will be higher than the density of pure water because the salt increases the mass without significantly increasing the volume.
Common Mistakes and How to Avoid Them
Several common mistakes can lead to incorrect conversions. Here are some crucial points to remember:
- Using the wrong density: Always double-check that you are using the correct density for the substance you are working with. The density can vary significantly depending on the temperature and purity of the substance.
- Incorrect unit conversions: Pay close attention to units and ensure consistent use of grams/kilograms and milliliters/liters throughout the calculation. Failing to convert between kilograms and grams or liters and milliliters is a common error.
- Not considering significant figures: When performing calculations, remember to consider the significant figures in your measurements to ensure the accuracy of your final answer.
- Assuming a density of 1 g/mL: This is only true for water (under specific conditions) and not for other substances. Always use the appropriate density value for the substance involved.
Frequently Asked Questions (FAQ)
Q1: Can I convert grams to liters directly without knowing the density?
A1: No, you cannot. The conversion requires knowing the density of the substance because it represents the relationship between mass and volume.
Q2: What if I have a mixture of substances? How do I calculate the density?
A2: Calculating the density of a mixture is more complex and involves considering the densities and proportions of each component in the mixture. Methods like weighted averages may be used, but the exact method depends on the nature of the mixture.
Q3: Why is density important in everyday life?
A3: Density plays a significant role in various everyday applications. For example, it determines whether an object will float or sink in water (buoyancy), influences the efficiency of engines (fuel density), and is essential in many industrial processes (e.g., material selection).
Q4: Are there online calculators for these conversions?
A4: Yes, many online calculators are available that can perform these conversions once you provide the necessary information (mass or volume, and density). However, understanding the underlying principles is crucial to avoid errors and build a solid scientific foundation.
Conclusion: Mastering Mass-Volume Conversions
Mastering the conversion between grams, milliliters, kilograms, and liters is a valuable skill in various scientific and practical applications. By understanding the concept of density and applying the relevant formulas, you can accurately convert between mass and volume units for any substance once you know its density. Remember to always double-check your units, use the correct density value, and consider the significant figures in your calculations to ensure accuracy. This detailed guide provides a strong foundation for performing these essential conversions with confidence. Remember that the key is understanding the fundamental relationship between mass, volume, and density. With practice and careful attention to detail, you can confidently navigate these conversions in your studies or everyday life.
Latest Posts
Latest Posts
-
Mass Density And Volume Relation
Sep 22, 2025
-
Spanish Words Starting With E
Sep 22, 2025
-
Is 2 Greater Than 1
Sep 22, 2025
-
Words With The Suffix Al
Sep 22, 2025
-
Objects That Begin With R
Sep 22, 2025
Related Post
Thank you for visiting our website which covers about G Ml To Kg L . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.