Enantiomers Are Molecules That _____.
seoindie
Sep 24, 2025 · 7 min read
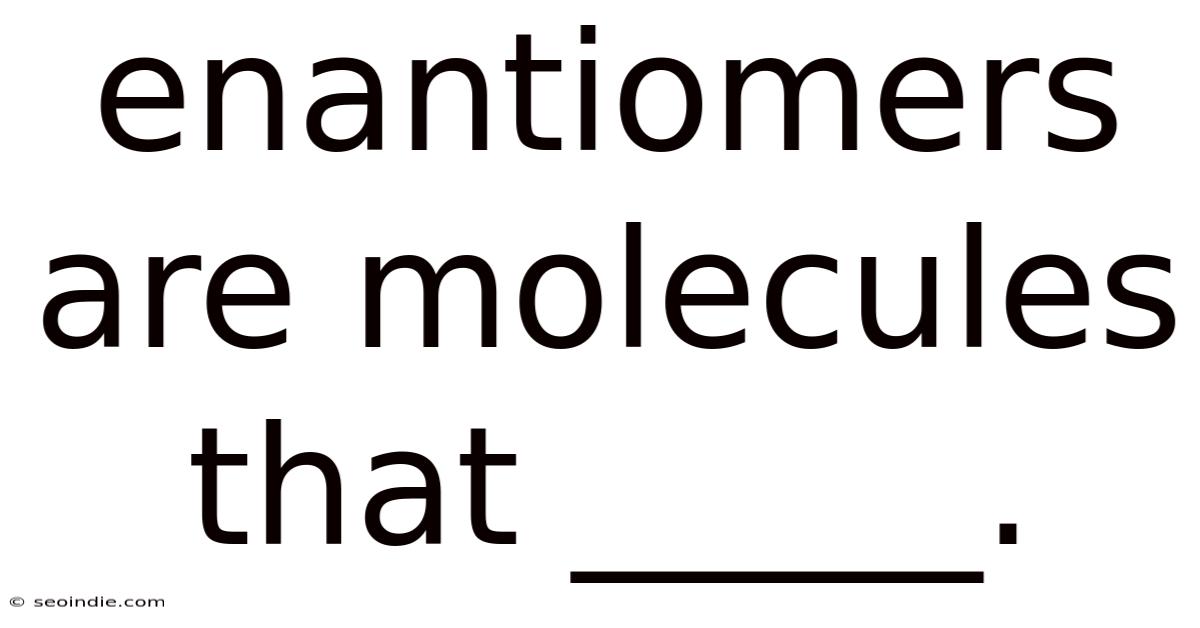
Table of Contents
Enantiomers Are Molecules That… Exhibit Chirality and Optical Activity
Enantiomers are molecules that are non-superimposable mirror images of each other. This seemingly simple definition belies a profound impact on chemistry, biology, and even pharmacology. Understanding enantiomers requires delving into the concept of chirality, optical activity, and the implications these properties have on various fields. This article will explore these concepts in detail, providing a comprehensive understanding of what makes enantiomers so unique and important.
Introduction to Chirality and Stereochemistry
Before diving into enantiomers, let's establish a firm understanding of chirality and stereochemistry. Stereochemistry is the branch of chemistry concerned with the three-dimensional arrangement of atoms and molecules and how this arrangement affects their chemical and physical properties. Chirality, derived from the Greek word "cheir" meaning hand, refers to a molecule's property of being non-superimposable on its mirror image. Think of your hands: they are mirror images, but you cannot overlay one perfectly onto the other. This same principle applies to chiral molecules.
A key feature of chiral molecules is the presence of a stereocenter or chiral center. This is typically a carbon atom bonded to four different groups. The arrangement of these groups around the stereocenter determines whether the molecule is chiral. If swapping any two groups leads to a different molecule (its mirror image), then the molecule is chiral and can exist as a pair of enantiomers.
Defining Enantiomers: Mirror Images with Different Properties
Enantiomers, then, are a specific type of stereoisomer. Stereoisomers are molecules with the same molecular formula and connectivity but different spatial arrangements of atoms. Enantiomers are a special subset of stereoisomers, distinguished by their mirror-image relationship. Crucially, they are not superimposable. No matter how you rotate or manipulate one enantiomer, you cannot make it identical to its mirror image.
While enantiomers have the same physical properties like melting point, boiling point, and solubility in achiral solvents (solvents that are not chiral themselves), they exhibit different properties in chiral environments. This is where their unique characteristics become apparent. One of the most significant differences lies in their interaction with plane-polarized light.
Optical Activity: The Twist of Light
Enantiomers possess the remarkable ability to rotate the plane of plane-polarized light. Plane-polarized light, unlike ordinary light, vibrates in only one plane. When plane-polarized light passes through a solution containing a chiral molecule, the plane of polarization is rotated either clockwise (dextrorotatory, denoted by + or d) or counterclockwise (levorotatory, denoted by - or l).
The extent of rotation is measured using a polarimeter and is expressed as the specific rotation, [α]. This value is specific to each enantiomer and depends on factors such as concentration, temperature, and wavelength of light used. Importantly, enantiomers rotate plane-polarized light by the same magnitude but in opposite directions. One enantiomer will rotate the light to the right, while its mirror image will rotate it to the left by the same degree. This difference in optical activity is a defining characteristic of enantiomers.
Racemic Mixtures: A 50/50 Blend
When a chiral molecule is synthesized in a non-chiral environment, usually both enantiomers are formed in equal amounts. This mixture of equal quantities of both enantiomers is called a racemic mixture or racemate. Because the rotations of the enantiomers cancel each other out, a racemic mixture does not rotate plane-polarized light. It is optically inactive.
Separating the enantiomers from a racemic mixture is a process known as resolution. Resolution techniques often involve using chiral resolving agents, which selectively interact with one enantiomer more strongly than the other, allowing for their separation. This separation is critical in many applications, as the different enantiomers can have significantly different biological activities.
Enantiomers in Biology and Medicine: A Tale of Two Molecules
The significance of enantiomers extends far beyond the realm of theoretical chemistry. In biology, many molecules, particularly biomolecules like amino acids and sugars, exist as enantiomers. Living organisms are highly selective in their interactions with chiral molecules. For example, most amino acids found in proteins are L-amino acids, while D-amino acids are rarely found. Similarly, the sugars in DNA and RNA are predominantly D-sugars. This chiral selectivity is essential for the proper functioning of biological systems.
In medicine, the difference between enantiomers can be dramatic. A classic example is thalidomide, a drug once used as a sedative. One enantiomer was effective in treating morning sickness, while the other caused severe birth defects. This tragic case highlighted the crucial importance of considering the chirality of drug molecules and developing enantiomerically pure drugs whenever possible. Many modern drugs are now designed and synthesized as single enantiomers to maximize therapeutic effects and minimize side effects. This is often a challenging and expensive process, requiring careful control of stereochemistry throughout the synthesis.
Naming Enantiomers: R and S Configurations
To distinguish between enantiomers, chemists use a system of nomenclature based on the Cahn-Ingold-Prelog (CIP) priority rules. This system assigns priorities to the four groups attached to a chiral center based on atomic number. Then, by visualizing the molecule with the lowest priority group pointing away from you, you determine the order of the remaining three groups. If the order is clockwise, the enantiomer is designated as R (rectus, Latin for right); if the order is counterclockwise, it is designated as S (sinister, Latin for left).
This R/S system provides a unambiguous way to describe the absolute configuration of a chiral molecule, allowing for clear communication between scientists and accurate representation in scientific literature.
Diastereomers: Non-mirror Image Stereoisomers
It's crucial to differentiate enantiomers from other types of stereoisomers, especially diastereomers. While both are stereoisomers, diastereomers are not mirror images of each other. They have different spatial arrangements, but this difference does not correspond to a mirror image relationship. Diastereomers exhibit different physical and chemical properties, including different optical rotations. For example, cis-trans isomers are a type of diastereomer. The differences between diastereomers are often more significant than those between enantiomers.
Meso Compounds: Achiral Molecules with Chiral Centers
Another important concept is that of meso compounds. Meso compounds possess chiral centers but are overall achiral due to internal symmetry. This means they are superimposable on their mirror images. Although they contain chiral centers, the symmetry cancels out their optical activity, making them optically inactive.
Frequently Asked Questions (FAQs)
Q: Are all chiral molecules optically active?
A: Generally, yes. However, meso compounds are exceptions. They possess chiral centers but are optically inactive due to internal symmetry.
Q: How can I determine if a molecule is chiral?
A: Look for the presence of a stereocenter (usually a carbon atom bonded to four different groups). If the molecule is non-superimposable on its mirror image, it is chiral.
Q: What is the significance of enantiomer purity in pharmaceuticals?
A: Enantiomer purity is crucial because different enantiomers can have vastly different biological activities. One enantiomer might be therapeutically active, while the other might be inactive or even toxic.
Q: How are enantiomers separated?
A: Enantiomer separation, or resolution, is often achieved through techniques such as chiral chromatography or by reacting the racemic mixture with a chiral resolving agent.
Q: What are the applications of enantiomer separation?
A: Enantiomer separation is essential in various fields, including pharmaceuticals, where only one enantiomer might be therapeutically active, and food science, where the different enantiomers of a flavoring compound might have different tastes.
Conclusion: The Importance of Chirality
Enantiomers are molecules that are non-superimposable mirror images of each other, exhibiting chirality and often optical activity. Their existence has profound implications across various scientific disciplines, particularly in biology and medicine. The selective interactions of biological systems with specific enantiomers, as well as the often vastly different effects of enantiomers in pharmacology, underscore the importance of understanding and controlling chirality in numerous applications. From drug development to the study of biological systems, the understanding of enantiomers remains a fundamental aspect of modern chemistry and its related fields. The ability to synthesize and separate enantiomers allows for the creation of more effective and safer medicines, contributing significantly to advancements in healthcare and scientific discovery. Further research in this area continues to expand our understanding of the intricate role of chirality in the natural world and its technological applications.
Latest Posts
Latest Posts
-
Cubic Foot To Cubic Inches
Sep 24, 2025
-
Convert 12 5 Inches To Cm
Sep 24, 2025
-
Proof Of Isosceles Triangle Theorem
Sep 24, 2025
-
Plant With Multi Colored Leaves
Sep 24, 2025
-
E S I Full Form
Sep 24, 2025
Related Post
Thank you for visiting our website which covers about Enantiomers Are Molecules That _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.