Cu Oh 2 Molar Mass
seoindie
Sep 12, 2025 · 5 min read
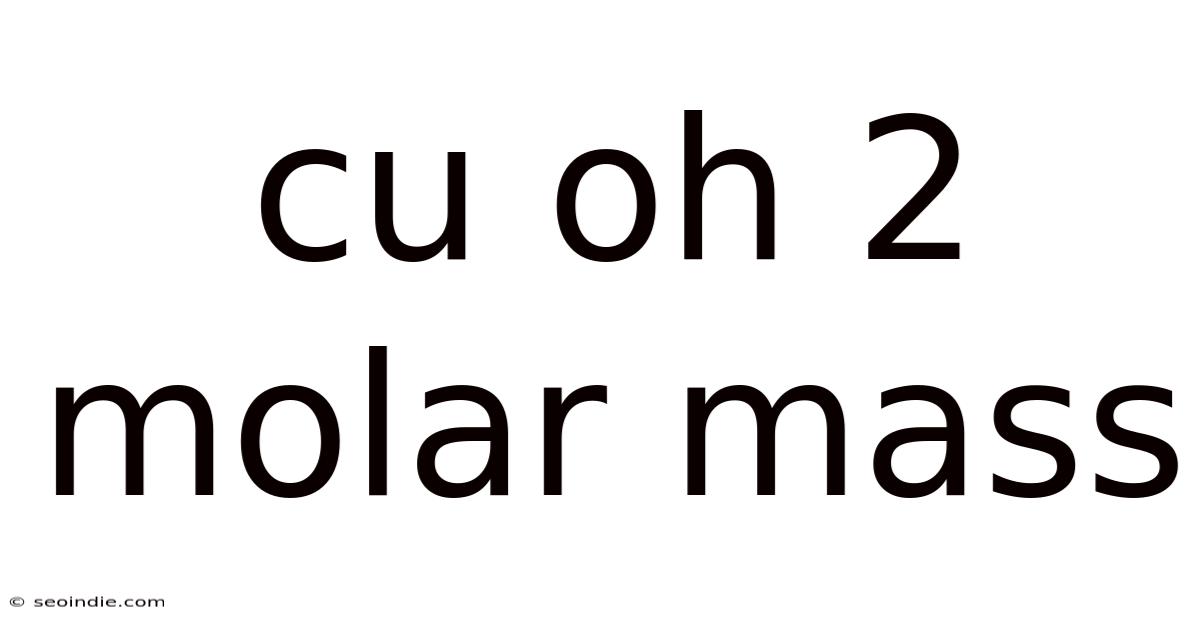
Table of Contents
Understanding the Molar Mass of Cu(OH)₂: A Deep Dive into Copper(II) Hydroxide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article delves into the calculation and understanding of the molar mass of copper(II) hydroxide, Cu(OH)₂, exploring its chemical properties, applications, and significance. We'll cover the step-by-step calculation, address common misconceptions, and provide additional insights into related chemical concepts. This guide aims to equip you with a thorough understanding of Cu(OH)₂'s molar mass and its broader implications in chemistry.
Introduction: What is Molar Mass?
Before we delve into the specifics of Cu(OH)₂, let's clarify the concept of molar mass. Molar mass is the mass of one mole of a substance. A mole is a unit representing a specific number of particles, namely Avogadro's number (approximately 6.022 x 10²³). Therefore, the molar mass essentially tells us the mass of 6.022 x 10²³ atoms, molecules, or formula units of a substance, expressed in grams per mole (g/mol).
Calculating the Molar Mass of Cu(OH)₂: A Step-by-Step Guide
To calculate the molar mass of Cu(OH)₂, we need to consider the atomic masses of its constituent elements: copper (Cu), oxygen (O), and hydrogen (H). These atomic masses are typically found on the periodic table. The values used here are approximate:
- Copper (Cu): 63.55 g/mol
- Oxygen (O): 16.00 g/mol
- Hydrogen (H): 1.01 g/mol
Step 1: Identify the number of atoms of each element:
In one formula unit of Cu(OH)₂, we have:
- 1 copper (Cu) atom
- 2 oxygen (O) atoms
- 2 hydrogen (H) atoms
Step 2: Calculate the mass contribution of each element:
- Copper: 1 Cu atom × 63.55 g/mol = 63.55 g/mol
- Oxygen: 2 O atoms × 16.00 g/mol = 32.00 g/mol
- Hydrogen: 2 H atoms × 1.01 g/mol = 2.02 g/mol
Step 3: Sum the mass contributions:
Add the mass contributions of each element to obtain the molar mass of Cu(OH)₂:
63.55 g/mol + 32.00 g/mol + 2.02 g/mol = 97.57 g/mol
Therefore, the molar mass of Cu(OH)₂ is approximately 97.57 g/mol. This means that one mole of Cu(OH)₂ weighs approximately 97.57 grams.
Understanding the Significance of Molar Mass
Knowing the molar mass of Cu(OH)₂ is essential for various chemical calculations, including:
- Stoichiometry: Determining the amounts of reactants and products in chemical reactions. For example, if you are reacting Cu(OH)₂ with another substance, you need its molar mass to calculate the appropriate quantities for a balanced reaction.
- Concentration Calculations: Determining the concentration of solutions. For instance, preparing a solution of a specific molarity (moles per liter) requires knowing the molar mass of the solute (Cu(OH)₂ in this case).
- Gravimetric Analysis: Analyzing the mass of a precipitate to determine the amount of a specific substance in a sample. If Cu(OH)₂ is involved as a precipitate, its molar mass is crucial for quantitative analysis.
Applications of Copper(II) Hydroxide
Cu(OH)₂ finds applications in various industries and processes:
- Pigments: Cu(OH)₂ is used as a pigment in paints and coatings, providing a characteristic blue-green color.
- Fungicides: Due to its antimicrobial properties, it is utilized as a fungicide in agriculture.
- Catalysis: It can act as a catalyst in some chemical reactions.
- Production of other copper compounds: It serves as a precursor in the synthesis of other copper-containing compounds.
Common Misconceptions about Molar Mass Calculations
A common mistake is neglecting to account for the number of atoms of each element in the chemical formula. Always carefully examine the subscript numbers in the formula to determine the correct number of atoms for each element before calculating the molar mass.
Explanation of Chemical Properties: Cu(OH)₂
Copper(II) hydroxide is a light blue-green, gelatinous solid. It is sparingly soluble in water but readily soluble in acids and ammonia solutions. It is an amphoteric substance, meaning it can react with both acids and bases. Its chemical properties are largely influenced by the presence of the hydroxide (OH⁻) ion, which is a strong base. This makes Cu(OH)₂ reactive towards acids, readily undergoing neutralization reactions.
Further Exploration: Related Chemical Concepts
Understanding molar mass lays the foundation for grasping more advanced chemical concepts, such as:
- Avogadro's Law: This law states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. This is directly related to the concept of moles and molar mass.
- Stoichiometric Calculations: These calculations involve using balanced chemical equations and molar masses to determine the quantities of reactants and products in a chemical reaction.
- Acid-Base Chemistry: The amphoteric nature of Cu(OH)₂ and its reactions with acids and bases highlight the importance of acid-base chemistry.
Frequently Asked Questions (FAQ)
-
Q: What is the difference between molar mass and molecular weight?
- A: The terms are often used interchangeably. Molecular weight generally refers to the mass of a single molecule, while molar mass refers to the mass of one mole of molecules.
-
Q: How accurate is the molar mass calculation using approximate atomic masses?
- A: Using approximate atomic masses from the periodic table provides a sufficiently accurate result for most purposes. However, for highly precise calculations, more accurate atomic masses may be needed.
-
Q: Can the molar mass of Cu(OH)₂ vary?
- A: The molar mass will slightly vary depending on the isotopic composition of copper, but the value calculated using the average atomic mass is sufficient for most applications.
-
Q: What are some practical applications of understanding molar mass calculations?
- A: Practical applications include determining the concentration of solutions, calculating the yield of chemical reactions, and analyzing the composition of substances.
Conclusion: Molar Mass – A Cornerstone of Chemistry
Understanding the molar mass of Cu(OH)₂, and molar mass calculations in general, is a critical aspect of chemistry. It enables us to perform various quantitative analyses, predict reaction yields, and comprehend the stoichiometry of chemical reactions. This concept acts as a bridge between the microscopic world of atoms and molecules and the macroscopic world of observable quantities. Mastering this fundamental concept is crucial for success in chemistry and related fields. The detailed explanation and practical examples provided here should equip you with the knowledge and confidence to tackle more complex chemical calculations involving molar masses. Remember to always refer to a periodic table for the most up-to-date atomic masses for the most accurate results.
Latest Posts
Latest Posts
-
180 Meters Squared In Feet
Sep 12, 2025
-
3 Km How Many Meters
Sep 12, 2025
-
Motivational Words Start With E
Sep 12, 2025
-
Verbs That Start With N
Sep 12, 2025
-
What Is Half Of 58
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Cu Oh 2 Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.